
For instance, lithium ( Li \text Ne start text, N, e, end text ), on the other hand, has a total of ten electrons: two are in its innermost 1 s 1s 1 s 1, s orbital and eight fill the second shell-two each in the 2 s 2s 2 s 2, s and three p p p p orbitals, 1 s 2 1s^ 2 1 s 2 1, s, squared 2 s 2 2s^ 2 2 s 2 2, s, squared 2 p 6 2p^6 2 p 6 2, p, start superscript, 6, end superscript. Elements in the second row of the periodic table place their electrons in the 2n shell as well as the 1n shell. After the 1 s 1s 1 s 1, s orbital is filled, the second electron shell begins to fill, with electrons going first into the 2 s 2s 2 s 2, s orbital and then into the three p p p p orbitals.
Gold in element chart plus#
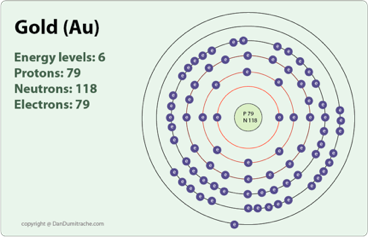
The second electron shell, 2n, contains another spherical s s s s orbital plus three dumbbell-shaped p p p p orbitals, each of which can hold two electrons. Hydrogen and helium are the only two elements that have electrons exclusively in the 1 s 1s 1 s 1, s orbital in their neutral, non-charged, state. On the periodic table, hydrogen and helium are the only two elements in the first row, or period, which reflects that they only have electrons in their first shell. This is written out as 1 s 2 1s^ 2 1 s 2 1, s, squared, referring to the two electrons of helium in the 1 s 1s 1 s 1, s orbital. The IUPAC defines a transition metal as an element that has a partly filled subshell, or an atom that may give rise to cations despite having an. Gold falls under the sixth period and group eleven in the periodic table. Helium has two electrons, so it can completely fill the 1 s 1s 1 s 1, s orbital with its two electrons. Gold is a precious metallic element with atomic number 79 and is a part of the periodic table. This can be written out in a shorthand form called an electron configuration as 1 s 1 1s^ 1 1 s 1 1, s, start superscript, 1, end superscript, where the superscripted 1 refers to the one electron in the 1 s 1s 1 s 1, s orbital. Hydrogen has just one electron, so it has a single spot in the 1 s 1s 1 s 1, s orbital occupied. The 1 s 1s 1 s 1, s orbital is the closest orbital to the nucleus, and it fills with electrons first, before any other orbital. It can be sobering to realize how much impact a single element can have on life-or the ease with which human activity can affect the environment.Įach element has a name.The first electron shell, 1n, corresponds to a single 1 s 1s 1 s 1, s orbital. You may even see statements to that effect on detergent boxes. Today, many detergents are made without phosphorus so the detrimental effects of eutrophication are minimized. This process, called eutrophication, is considered a negative environmental impact. Because of the large bacterial concentrations, the oxygen content of the water dropped, causing fish to die in large numbers. When the algae died, concentrations of bacteria that ate the dead algae increased. Lakes receiving this wastewater experienced sudden increases in growth of algae. When phosphorus-containing detergents were introduced in the 1950s, wastewater from normal household activities greatly increased the amount of phosphorus available to algae and other plant life. Higher forms of life, such as humans, can obtain phosphorus by selecting a proper diet (plenty of protein) but lower forms of life, such as algae, must absorb it from the environment. Its availability limits the amount of life our planet can sustain. Phosphorus, then, is nature’s bottleneck. Unlike carbon, which can be obtained from carbon dioxide, there is no phosphorus compound present in our surroundings that can serve as a convenient source. Use the buttons above to change your view of the periodic table and view Murray Robertson’s stunning Visual Elements artwork. Click the tabs at the top to explore each section. We need phosphorus for our bones and teeth, and it is a crucial component of all living cells. The Royal Society of Chemistrys interactive periodic table features history, alchemy, podcasts, videos, and data trends across the periodic table. Phosphorus makes up 1.1% of the human body but only 0.105% of Earth’s crust. There is an element that we need more of in our bodies than is proportionately present in Earth’s crust, and this element is not easily accessible. LOOKING CLOSER: PHOSPHOROUS, THE CHEMICAL BOTTLENECK On the other hand, although carbon is present in the atmosphere as carbon dioxide, and about 80% of the atmosphere is nitrogen, we obtain those two elements from the food we eat, not the air we breathe. We obtain oxygen from the air we breathe and the water we drink. The relative amounts of elements in the body have less to do with their abundances on Earth than with their availability in a form we can assimilate.
CRC Handbook of Chemistry and Physics, 89th ed. \): Elemental Composition of a Human Body Element


 0 kommentar(er)
0 kommentar(er)
